Abstract
Langerhans cell histiocytosis (LCH) represents a disorder characterised by an abnormal accumulation of histiocytes in miscellaneous tissues. The bone is commonly affected, especially the flat bones, the spine and the long bones. Some lesions in children such as a “vertebra plana” or a solitary lytic lesion of the skull may be suggestive for LCH, whereas others can be confused with a malignant tumour or osteomyelitis. This pictorial essay presents the main usual and unusual skeletal manifestations observed in LCH.
Teaching points
• Osseous involvement in children with LCH is very similar to that seen in multiple myeloma.
• A solitary lytic lesion of the cranial vault is a typical radiographic finding of LCH.
• A vertebra plana appearance in the spine is another typical radiographic finding.
• Extensive signal intensity changes within bone marrow on MRI are a helpful sign for the diagnosis.
• In long bones, endosteal scalloping may be responsible for a “budding appearance”.
Similar content being viewed by others
Introduction
Langerhans cell histiocytosis (LCH) represents a spectrum of rare disorders characterised by idiopathic infiltration and accumulation of abnormal histiocytes (i.e. the Langerhans cells) within various tissues (bone marrow, skin, central nervous system, lung, liver, spleen, lymph nodes) causing focal or systemic effects [1]. LCH predominates in children and its annual incidence is estimated at 4.6 per million in children under 14 years of age [2].
LCH was formerly known as “histiocytosis X”, a term that grouped three major syndromes, which are now considered as clinical variants of the same disease: the eosinophilic granuloma (unifocal LCH with a solitary bone lesion), the Hand-Schüller-Christian disease (multifocal LCH with the classic triad of skull lesions, exophtalmos, and diabetes insipidus) and the Letterer-Siwe disease (fulminant LCH with multiple organ involvement) [3]. The aetiology of LCH remains unknown, and it is still uncertain whether LCH is a neoplastic disorder, suggested by the monoclonality in lesions, or a reactive disorder resulting from a dysregulation of the immune system [4, 5].
Clinical presentations of LCH vary widely, from an asymptomatic solitary bone lesion to a multisystem life-threatening affliction [1, 6, 7]. Any organ or system of the human body can be involved, but the skeleton, the skin and the central nervous system are more commonly affected [1, 6, 7]. Biopsy taken from the most easily accessible lesion (usually a skin or a soft tissue biopsy) is necessary to confirm the diagnosis. Histopathological examination shows tissue infiltration by abnormal Langerhans cells as well as normal inflammatory cells (T cells, eosinophils, macrophages and multinucleated giant cells) [1]. Diagnosis is confirmed by the morphologic identification of the Langerhans cells and positive immunohistochemical staining with CD1a and/or CD207. The identification of Birbeck granules on electron microscopy, which are cytoplasmic tennis-racket-shaped organelles characteristic of Langerhans cells, is nowadays rarely performed [6]. A bone biopsy is often not needed for the diagnosis of LCH, but it may be required in some cases. When the risk of such a biopsy outweighs the need for a definitive diagnosis (as this may be the case in some children with isolated upper cervical vertebral involvement), careful monitoring by clinical examination and appropriate imaging for at least the next 6 months is necessary in order to exclude a malignancy and to reassess the need for biopsy [7].
Once the diagnosis has been ascertained, the treatment depends on the extent and severity of LCH. Extent of the disease makes the distinction between single system disease and multisystem disease. Severity of the disease takes into account the involvement or not of risk organs (i.e. the haematological system, the spleen and the liver) and the presence or not of central nervous system risk lesions (i.e. skull bone lesions with the exception of the vault) [7]. Therefore, different therapeutic approaches may be applied; they include conservative management, local therapy (curettage, excision, intralesional corticosteroid injection, low-dose radiation therapy) and systemic therapy (chemotherapy, corticosteroids, stem cell transplantation) [4, 7].
Imaging techniques in assessment of skeletal involvement
Bone is the most frequently affected tissue in children with LCH, encountered in about 75–80% of patients with LCH [2, 7], with unifocal involvement being more common than multifocal involvement [4, 8, 9]. LCH can involve any bone, but there is a predilection for the axial skeleton, with more than 50% of bone lesions occurring in the flat bones (skull, ribs, pelvis) [3]. In the long bones, the femur is the most commonly affected, followed by the humerus and the tibia [3]. Imaging is based predominantly on radiography. In children with biopsy-proven LCH, a chest radiograph and complete skeletal survey have to be obtained for diagnosis and assessment of disease extent, in addition to laboratory evaluation [6]. In our institution, this survey is performed with a low-dose biplanar system whenever possible, because this new technique allows a quick assessment of the axial skeleton in the standing position (Fig. 1) with a reduced amount of radiation exposure (by a factor of 8–10) compared with conventional radiography [10–13]. However, this technique cannot be used in very young children unable to stand and its value has not been assessed yet in LCH. Whatever the technique used, the sensitivity of radiography is limited because lytic lesions become apparent only when 30–50% of the bone mineral density is already lost and because some areas such as the skull base, the scapulae, the spine and the pelvis may be difficult to assess owing to superposition of bones or bowel gas. Despite this, radiography remains the “gold standard” of the diagnostic and staging procedure. Once a bone lesion is diagnosed, computed tomography (CT) or magnetic resonance imaging (MRI) may be necessary to assess precisely the degree of trabecular and cortical bone destruction in areas at risk of impending fracture (Fig. 2) and to guide a bone biopsy if necessary (CT), or to assess the degree of soft tissue infiltration in areas at risk of neurological complications (MRI) (Fig. 3). Bone scintigraphy may be a complementary technique to the radiographic skeletal survey in assessment of bone involvement, but its sensitivity is limited as well, especially in the skull [14, 15], and this modality has radiation issues. Recently, new imaging techniques, such as positron emission tomography-computed tomography (PET-CT) and whole-body MRI, have developed in view of an improved assessment of the extent and severity of the disease [16–18]. Positron emission tomography-computed tomography (PET-CT) provides information related to disease activity and response to therapy [17, 18], but the inherent radiation burden is questionable. Whole-body MRI can detect extra-skeletal and skeletal lesions without use of ionising radiation (Fig. 4) [12], but its exact role in the diagnostic algorithm of LCH needs further investigation and access to this technique is still limited. Therefore, radiography still remains the screening technique of choice for the evaluation of children with suspected LCH.
a Coronal CT image of the left hip shows large, well-defined, lytic, femoral and acetabular lesions. The femoral lesion, at risk of impending fracture, was immobilised thereafter. Note the “budding appearance” of bone destruction (arrows) related to endosteal scalloping. b The diagram depicts this “budding appearance” in the diaphysis of a long bone. M medullary canal, C cortex
Imaging features of skeletal involvement
The radiographic appearance of the lesions depends on the site of involvement and the phase of the disease. The axial skeleton (skull, spine, scapula, and pelvis) and the long bones are more commonly affected. Bone involvement usually presents as a single or multiple osteolytic lesions, round or oval-shaped. Their border can appear either well or poorly defined (Fig. 5). Such an osseous involvement is very similar to that of multiple myeloma in adults. In LCH, however, periosteal reaction may be associated with osseous lesions. When present, periostitis demonstrates the appearance of a single layer of periosteal bone or a more suggestive buttressed pattern (Fig. 6). During the healing phase, sclerotic margins appear but sclerosis is usually limited. Lesions can eventually completely disappear, with no or little deformity (Fig. 7) [3]. CT and MR features are non-specific but an extensive bone marrow oedema is usually seen around bone lesions on MRI (Fig. 8).
Skull
Calvarium
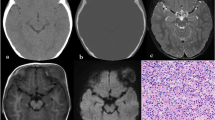
This is affected more often than the skull base, and lesions occur especially in the parietal or the frontal region [19]. Radiographically, they typically appear as one or multiple well-defined punched-out osteolytic lesions (Fig. 9). Unequal involvement of the inner and outer tables may give the lesions a characteristic bevelled-edge appearance on CT scans (Fig. 10). These lytic lesions may sometimes contain a fragment of intact bone, referred to as “button sequestrum” (Fig. 10). This sign, however, is not specific for LCH and can also be seen in other diseases such as osteomyelitis [20]. Multiple lesions may enlarge and coalesce, giving a “geographic” appearance to the skull vault. On MRI, calvarial lesions usually appear isointense at T1-weighted imaging, heterogeneously hyperintense at T2-weighted imaging, and present a marked enhancement after gadolinium injection. An associated soft-tissue mass or reactive dural enhancement can also be well evaluated on MRI [21]. In extremely rare cases, bleeding of LCH lesions may be responsible for an extradural haematoma (Fig. 11) [22]. The main differential diagnoses of calvarial lytic lesions on imaging include epidermoid and dermoid cysts for a solitary lesion and metastatic neuroblastoma for multiple lesions. Dermoid and epidermoid cysts are most commonly seen in midline locations, sometimes associated with a sinus tract; CT attenuation and MR signal intensity changes vary depending on cyst’s content (fat in dermoid cysts, fluid in epidermoid cysts) [23]. Skull vault metastases do not have well-defined bevelled edges, like in LCH, and may be associated with speculated (“hair-on-end”) periosteal reaction.
Skull base
The temporal bone is the most commonly affected part of the skull base. CT shows destructive bone lesions with associated soft-tissue masses involving the mastoid (Fig. 12), with the squamous portion and middle ear less affected [24, 25]. Involvement of the auditory ossicles and the internal ear is not very frequent despite the extensive bony destruction. Paralysis of the cranial nerves is also unusual, but can occur when the petrous apex is involved or when there is an extension to the central nervous system. The main differential diagnosis of temporal bone involvement on imaging is mastoiditis, but infection is usually not associated with extensive osseous destruction and contrast-enhanced CT or MR images may reveal an accompanying abscess. While recovering, the LCH lesions show early disappearance of the soft-tissue mass, followed by reossification and remodelling of the involved bone [25]. A less commonly affected area in the skull base is the sphenoid bone with involvement of the clivus, the sphenoid wings or the pituitary stalk. The latter may be involved isolatedly (Fig. 13) or in association with sphenoid bone involvement.
Mandible and maxilla
Panoramic radiographs may show severe alveolar bone destruction, which produces the appearance of “floating teeth” (Fig. 14) [26].
Orbit
This is another site of predilection for LCH that usually presents as an isolated bone lesion with an associated soft tissue mass, although it can also be encountered in multifocal or multisystemic disease. It occurs predominantly in the superior or superolateral orbital region (Fig. 15) [24, 27]. Complementary CT and/or MRI are useful to precisely determine the destruction of osseous structures and the extension into the orbit, and possibly the temporal fossa, forehead, and face [24].
Chest wall
Rib lesions are lytic (Fig. 16), sometimes associated with a pathological fracture. An extrapleural mass may result from soft-tissue extension [3]. Some rib lesions may be expansile (Fig. 16) or may appear aggressive, with a large soft tissue mass that may be mistaken in some cases for Ewing’s sarcoma. However, even if a bone biopsy is mandatory, the presence of extensive signal intensity changes around the lesion (Fig. 16) is more suggestive of LCH than Ewing’s sarcoma. The clavicle is less frequently involved in children but some cases of clavicular location have been reported in young adults (Fig. 17) [28, 29]. In contrast, scapular lesions are not uncommon in children and may appear expansile (Fig. 8) and/or aggressive.
a Chest radiograph reveals an expansile and lytic lesion of the lateral portion of a left rib (arrowhead). 16b Axial fat-suppressed T2-weighted MR image and sagittal fat-suppressed gadolinium-enhanced T1-weighted MR image show extensive bone marrow changes with soft tissue extension creating an extrapleural mass
Pelvis and spine
Pelvis
The iliac wings and the supra-acetabular region are more frequently involved. In the pelvis, bone lesions are not always purely lytic and may exhibit well-defined sclerotic margins (Fig. 18) [3].
a Anteroposterior radiograph of the pelvis reveals bilateral lytic lesions of the iliac wings. On the left, well-defined sclerotic margins are seen around the lesion that extends to the supra-acetabular region. b On axial CT scan, note the presence of a fragment of intact bone within the left bone lesion (arrow) with reactive sclerosis
Spine
In the spine, LCH has a predilection for the thoracic spine, followed by the lumbar spine and the cervical spine. In most cases, it involves the vertebral bodies with relative sparing of the posterior elements. Such involvement may result in anterior wedging or more commonly, in complete collapse with a characteristic vertebra plana appearance (Fig. 7) [9, 30]. With healing, partial (Fig. 7) or almost complete height restitution of the involved vertebra may be seen [3, 9]. In some patients, asymmetrical vertebral body collapse, involvement of the vertebral body as well as the posterior elements (Fig. 19), and much less frequently, lesions limited to the posterior element may be encountered (Fig. 20) [3]. Solitary lytic lesions in the cervical spine are very rare; they may be associated with spinal deformity (Fig. 21) or epidural soft-tissue extension that is well-depicted on MRI (Fig. 3). However, spinal cord compression is extremely rare [31].
a Axial CT scan demonstrates an ill-defined osteolysis of a thoracic vertebra involving the vertebral body but also the right pedicle and the transverse process. b Sagittal fat-suppressed gadolinium-enhanced T1-weighted MR image shows the degree of epidural extension. The collapsed vertebral body is evident
Long bones
In the long bones, LCH initially presents as ill-defined areas of trabecular and/or cortical bone destruction, usually involving the diaphysis (Fig. 5), the metaphysis or both. When a lesion involves the metaphysis, it does not usually cross the growth plate (Fig. 22). Thickening of the cortical bone and periosteal reaction (Fig. 6) may be associated. Lesions may later become well defined, and sclerotic margins may appear. Endosteal scalloping responsible for a “budding appearance” on CT and MRI (Figs. 2 and 23) [28], as well as extensive signal intensity changes within the bone marrow (Fig. 23), the periosteum and the adjacent soft tissues, may be helpful signs for the diagnosis of LCH. Involvement of the epiphysis is rare; a solitary epiphyseal lesion should thus suggest differential diagnoses such as chondroblastoma or epiphyseal osteomyelitis [19]. Transphyseal extension of LCH is also unusual and may result in premature closing of the physis. Other unusual features of LCH have been reported such as isolated cortical lesions, soft-tissue calcifications, extraskeletal soft tissue masses, fluid-fluid levels, and involvement of the short tubular bones of hands and feet [19].
a Coronal CT scan (22a) demonstrates an osteolytic lesion in the metaphysis of the right femur, without involvement of the epiphysis. Note the persistence of tiny bone fragments within the lesion. b Coronal unenhanced T1- and (c) fat-suppressed gadolinium-enhanced T1-weighted MR images show non-specific but extensive signal intensity changes within the bone marrow and the adjacent soft tissues as well as reactive synovitis
a Coronal fat-suppressed T2-weighted MR image reveals focal bone marrow replacement within the femoral diaphysis with a “budding” appearance due to endosteal scalloping and extensive oedema within the bone marrow and the adjacent soft tissues. b Axial T2-weighted MR image in another patient shows circumferential periosteal reaction (arrows) and extensive bone marrow oedema within the bone marrow and the adjacent soft tissues (asterisks)
Conclusion
LCH is a rare disorder, mostly affecting children. Osseous involvement in children with LCH is very similar to that seen in adults with multiple myeloma (i.e. predominance of destructive lesions in axial skeleton). It is best assessed with conventional or digital skeletal surveys. Radiographic features are variable, from a suggestive appearance to a more aggressive one, which may mimic Ewing’s sarcoma or osteomyelitis. Radiography may be complemented by CT and/or MRI to delineate the extent of osseous destruction or the extent of bone marrow and soft tissue involvement respectively. MR findings are non-specific, but extensive bone marrow changes and/or a “budding appearance” of bone lesions may be suggestive of the diagnosis. Biopsy is necessary to obtain diagnosis confirmation.
References
Windebank K, Nanduri V (2009) Langerhans cell histiocytosis. Arch Dis Child 94:904–908
Guyot-Goubin A, Donadieu J, Barkaoui M, Bellec S, Thomas C, Clavel J (2008) Descriptive epidemiology of childhood Langerhans cell histiocytosis in France, 2000–2004. Pediatr Blood Cancer 51:71–75
Stull MA, Kransdorf MJ, Devaney KO (1992) Langerhans cell histiocytosis of bone. Radiographics 12:801–823
Weitzman S, Egeler RM (2008) Langerhans cell histiocytosis: update for the pediatrician. Current Opin Pediatr 20:23–29
Leonidas JC, Guelfguat M, Valderrama E. Langerhans’ cell histiocytosis (203) Lancet 361:1293–1295
Satter EK, High WA (2008) Langerhans cell histiocytosis: a review of the current recommendations of the Histiocyte Society. Pediatr Dermatol 25(29):1–295
Haupt R, Minkov M, Astigarraga I, Schäfer E, Nanduri V, Jubran R, Egeler RM, Janka G, Micic D, Rodriguez-Galindo C, Van Gool S, Visser J, Weitzman S, Donadieu J, Euro Histio Network (2013) Langerhans cell histiocytosis (LCH): guidelines for diagnosis, clinical work-up, and treatment for patients till the age of 18 years. Pediatr Blood Cancer 60:175–184
The French Langerhans’ Cell Histiocytosis Study Group (1996) A multicentre retrospective survey of Langerhans’ cell histiocytosis: 348 cases observed between 1983 and 1993. Arch Dis Child 75:17–24
Azouz EM, Saigal G, Rodriguez MM, Podda A (2005) Langerhans’ cell histiocytosis: pathology, imaging and treatment of skeletal involvement. Pediatr Radiol 35:103–115
Dubousset J, Charpak G, Skalli W, Kalifa G, Lazennec JY (2007) EOS stereo-radiography system: whole-body simultaneous anteroposterior and lateral radiographs with very low radiation dose. Rev Chir Orthop Reparatrice Appar Mot 93:141–143
Deschênes S, Charron G, Beaudoin G, Labelle H, Dubois J, Miron MC, Parent S (2010) Diagnostic imaging of spinal deformities: reducing patients radiation dose with a new slot-scanning X-ray imager. Spine 35:989–994
McKenna C, Wade R, Faria R, Yang H, Stirk L, Gummerson N, Sculpher M, Woolacott N (2012) EOS 2D/3D X-ray imaging system: a systematic review and economic evaluation. Health Technol Assess 16:1–188
Gheno R, Nectoux E, Herbaux B, Baldisserotto M, Glock L, Cotten A, Boutry N (2012) Three-dimensional measurements of the lower extremity in children and adolescents using a LDBX-ray device. Eur Radiol 22:765–771
Dogan AS, Conway JJ, Miller JH, Grier D, Bhattathiry MM, Mitchell CS (1996) Detection of bone lesions in Langerhans cell histiocytosis: complementary roles of scintigraphy and conventional radiography. J Pediatr Hematol Oncol 18:51–58
Howarth DM, Mullan BP, Wiseman GA, Wenger DE, Forstrom LA, Dunn WL (1996) Bone scintigraphy evaluated in diagnosing and staging Langerhans’ cell histiocytosis and related disorders. J Nucl Med 37:1456–1460
Goo HW, Yang DH, Ra YS, Song JS, Im HJ, Seo JJ, Ghim T, Moon HN (2006) Whole-body MRI of Langerhans cell histiocytosis: comparison with radiography and bone scintigraphy. Pediatr Radiol 36:1019–1031
Binkovitz LA, Olshefski RS, Adler BH (2003) Coincidence FDG-PET in the evaluation of Langerhans’ cell histiocytosis: preliminary findings. Pediatr Radiol 33:598–602
Kaste SC, Rodriguez-Galindo C, McCarville ME, Shulkin BL (2007) PET-CT in pediatric Langerhans cell histiocytosis. Pediatr Radiol 37:615–622
Hindman BW, Thomas RD, Young LW, Yu L (1998) Langerhans cell histiocytosis: unusual skeletal manifestations observed in thirty-four cases. Skeletal Radiol 27:177–181
Krasnokutsky MV (2005) The button sequestrum sign. Radiology 236:1026–1027
Okamoto K, Ito J, Furusawa T, Sakai K, Tokiguchi S (1999) Imaging of calvarial eosinophil granuloma. Neuroradiology 41:723–728
Mut M, Cataltepe O, Bakar B, Cila A, Akalan N (2004) Eosinophilic granuloma of the skull associated with epidural haematoma: a case report and review of the literature. Childs Nerv Syst 20:765–769
Morón FE, Morriss MC, Jones JJ, Hunter JV ((2004) R) Lumps and bumps on the head in children: use of CT and MR imaging in solving the clinical diagnostic dilemma. Radiographics 24:1655–1674
D’Ambrosio N, Soohoo S, Warshall C, Johnson A, Karimi S (2008) Craniofacial and intracranial manifestations of Langerhans cell histiocytosis: report of findings in 100 patients. AJR Am J Roentgenol 191:589–597
Fernández-Latorre F, Menor-Serrano F, Alonso-Charterina S, Arenas-Jiménez J (2000) Langerhans’ cell histiocytosis of the temporal bone in pediatric patients: imaging and follow-up. AJR Am J Roentgenol 174:217–221
Eckardt A, Schultze A (2003) Maxillofacial manifestations of Langerhans cell histiocytosis: a clinical and therapeutic analysis of 10 patients. Oral Oncol 39:687–694
Herwig MC, Wojno T, Zhang Q, Grossniklaus HE (2012) Langerhans cell histiocytosis of the orbit: five clinicopathologic cases and review of the literature. Surv Ophthalmol. doi:10.1016/j.survophthal.2012.09.004
Song YS, Lee IS, Yi JH, Cho KH, Kim do K, Song JW (2011) Radiologic findings of adult pelvis and appendicular skeletal Langerhans cell histiocytosis in nine patients. Skeletal Radiol 40:1421–1426
Verbist B, Geusens E, Brys P, Verslegers I, Samson I, Sciot R, Baert AL (1998) Langerhans cell histiocytosis of the clavicle: a case report. Eur Radiol 8:1357–1358
Yeom JS, Lee CK, Shin HY, Lee CS, Han CS, Chang H (1999) Langerhans’ cell histiocytosis of the spine. Analysis of twenty-three cases. Spine 24:1740–1749
Jang KS, Jung YY, Kim SW (2010) Langerhans cell histiocytosis causing cervical myelopathy in a child. J Korean Neurosurg Soc 47:458–460
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Khung, S., Budzik, JF., Amzallag-Bellenger, E. et al. Skeletal involvement in Langerhans cell histiocytosis. Insights Imaging 4, 569–579 (2013). https://doi.org/10.1007/s13244-013-0271-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13244-013-0271-7